Getting the Word Out: Help Shape Our Plan for Peer-Reviewing and Making Our Primary Research Results Publicly Available
At PCORI, our mandate is to both support comparative clinical effectiveness research (CER) and get the results of those studies out to those who need them. It’s a task we take seriously as we build a body of funded research focused on helping patients and those who care for them make better-informed decisions about the health and healthcare choices they face.
The law that authorized our creation set two tests we must meet as our funded research starts to produce findings of interest to patients, clinicians, payers, employers, industry, and other stakeholders. First, we must peer-review our primary research—that is, ask appropriate experts to assess the results for scientific integrity and adherence to our Methodology Standards.
Second, we must make the results available within a specific time period—no later than 90 days from the “conduct or receipt” of the research findings—in a form understandable and useful to patients, clinicians, and the general public.
This is a substantial challenge, but one we’re excited to embrace as part of our commitment to fund research that will answer questions important to patients and improve care and outcomes. And to be sure we do this most effectively, we need your help.
We’ve just announced and posted a draft plan for how we will meet our obligation to peer-review our research and make the results publicly available, and we welcome your input during the official public comment period, which runs through 11:59 pm (ET) on November 7.
You can provide comments through our website. We’ll review all comments, analyze them for themes and trends, and then revise our proposal as needed to address the feedback. You can see all comments on our website shortly after they’re submitted, and we’ll provide a full report on how we addressed them when we update the proposal for our Board to consider for approval early next year.
We’ve already started hearing from many of our stakeholders, both individuals and organizations. We held a public forum on our draft plan in Washington, DC, on September 29; about 150 people attended in person and via webcast. We will hold a public webinar on October 29 as another opportunity for you to learn about what we propose.
Our Unique Charge and How We’re Trying to Meet It
All research funders receive reports summarizing findings from the studies they support, and many make some version of this information available to interested audiences. In addition, there are cases where government agencies that fund research are required to subject the work they support to peer review. But we think we’re in something of an unusual position when it comes to our dual obligations in these areas.
In addressing these obligations, we were mindful of the interests that different stakeholders have in this process and how those interests might, if not conflict, at least intersect in challenging ways. We’re counting on your comments to guide us as we finalize a process that, we hope, will balance these complexities in a way that will serve all stakeholders well.
We heard a range of thoughts on some of these issues during our public forum, which featured two panels of representatives of the patient, caregiver, research, publishing, clinician, payer, and industry communities. Just a few of the issues addressed:
- The law doesn’t say whether we should peer-review our primary research before making the results public. That’s the sequence we propose because we think assessing these studies for scientific and methodological rigor will give audiences confidence in the results. But review takes time. Is it worth the wait, given patient, caregiver, and clinician interest in the results?
- Researchers count on publishing their results in professional journals as a way to officially communicate with peers and others about their work. Those journals conduct their own peer review to determine which papers to accept. What happens if that review process and ours come to different conclusions?
- Many journals won’t publish a paper if the main results already have been made public in detail in some other way. We think our proposal addresses this issue, but others might not.
- We propose several means by which we’d make the results of our funded research available—an abstract for professionals, a version of the same abstract for patients and the general public, a table listing study results, and additional information about the awardee's affiliations and any conflicts of interest. Does that seem adequate? If not, what might we do instead?
- What specific factors should we keep in mind in ensuring that what we provide for patients and the general public meets their needs?
- When should we make public the final, peer-reviewed versions of the detailed project reports our funded researchers provide to us? How about the underlying sets of data that they collect?
It's Not Just about the Law
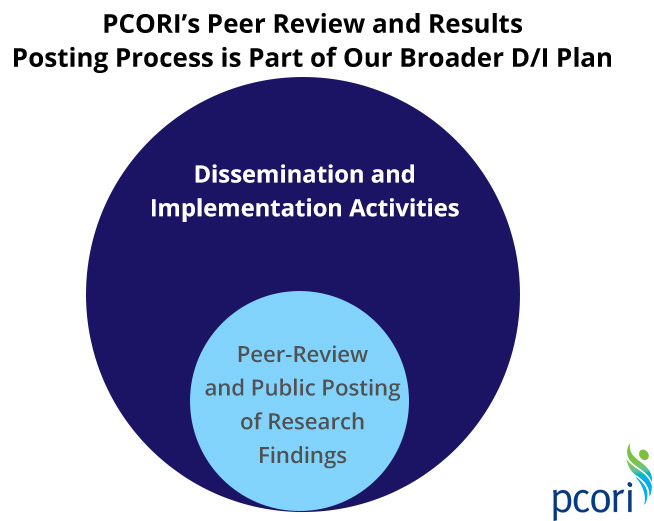
We consider our obligation to ensure the quality and broad availability of our research results to be more than a legal requirement. Explaining to you how we’re spending the dollars entrusted to us is a central commitment at PCORI. So are funding high-quality, useful research that answers patients’ questions and getting the results out broadly to quickly find their way into practice.
We view our proposal as part of a much wider dissemination and implementation plan that we are developing in close collaboration with the Agency for Healthcare Research and Quality, which is named in our authorizing legislation as our primary dissemination partner. This plan will help us assess the most effective ways to determine which of the findings of our funded research should be disseminated to which audiences, and how to best do that. We want to widely promote—well beyond our initial effort for making such results publicly available—findings that have strong potential to change clinical practice and health outcomes. We will team with patient advocacy and consumer organizations, as well as medical societies, healthcare systems, insurers, and others, to do that.
We’ve scheduled a public workshop this December to unveil our Dissemination and Implementation Framework and Toolkit, seeking input from a wide range of stakeholders on our proposed approach. We hope you’ll participate via webinar.
In the meantime, please take advantage of the public comment period for our proposed peer review and public release process. We’ll be analyzing the formal comments we receive in December and January, and we plan to provide our Board an updated version of our proposal, with any revisions resulting from the feedback we receive, for consideration for approval in late February.
Learn more about PCORI's proposed process for peer review and public release of its primary research findings at our October 29 webinar. Register here.