How PCORI Chooses Research Questions to Fund
PCORI has a broad charge: to fund research that improves the quality and relevance of evidence available to all healthcare stakeholders to help them make informed decisions. But such a goal translates into the difficult task of choosing our funding opportunities from a wide spectrum of potential research questions—What’s the best treatment for hepatitis C infection? How can we reduce cardiovascular disease risk in underserved populations? Which care management strategy improves quality of life for patients with dementia and decreases caregiver burden?
We are often asked how we decide which topics are worthy of study with PCORI support. We’ve also heard from many interested members of the healthcare community that they’d like to be able to track the topics and questions we’re considering for future funding opportunities. So we’re pleased to provide you an update in both these areas.
Focusing our Funding
 Click to enlarge
Click to enlarge
All of the studies we fund fit under one or more of our five National Priorities for Research. We solicit funding applications for that research through two main paths—announcements that ask for the research community’s best ideas for studies of topics within those broad topics and calls for proposals to study specific topics of particular interest to patients, clinicians, and other members of the diverse healthcare community we serve. This second path results both in individual “targeted” funding announcements focused on particular high-impact topics, such as obesity treatments for underserved populations, strategies to prevent older persons’ injuries from falls, and coordinated care for patients as they transition between care settings, such as from hospital to home. This path also leads to topics being called out for special attention through our Pragmatic Clinical Studies program, which funds projects that are often large and tend to be conducted in routine clinical settings.
Once we receive research proposals, our merit review panel provides another opportunity for stakeholder guidance, as patients, researchers, and other healthcare stakeholders score the applications against criteria that include potential to improve health care and outcomes in ways that are important to patients. Projects that make the grade then go to our multi-stakeholder Board of Governors for approval.
To be sure our largest investments go to research questions that matter most to the healthcare community, and in keeping with the intent of Congress as expressed in our enabling legislation, we use a systematic topic generation and research prioritization process that begins with questions suggested by patients and other stakeholders and keeps them involved on an ongoing basis.
We have recently enhanced the online description of our system for selecting such priority research questions. On our website, we now post lists of topics at each stage in the process so that stakeholders can comment on potential research questions throughout their development, and research teams can start thinking about likely topics for funding before we formally announce them.
Starting the Process
In selecting topics, our goal is to identify patient-centered comparative clinical effectiveness research (CER) questions that matter to our stakeholders but have not yet been reliably answered by previous studies nor are they being addressed by current research. We’re interested in questions that compare the effectiveness of two or more strategies for prevention, treatment, screening, diagnosis, or management of a condition or compare alternative system-level approaches; or compare factors that may affect patients' adherence to treatments. We also fund research that would help address disparities in health care, improve the communication of research findings, or advance methods for patient-centered CER.
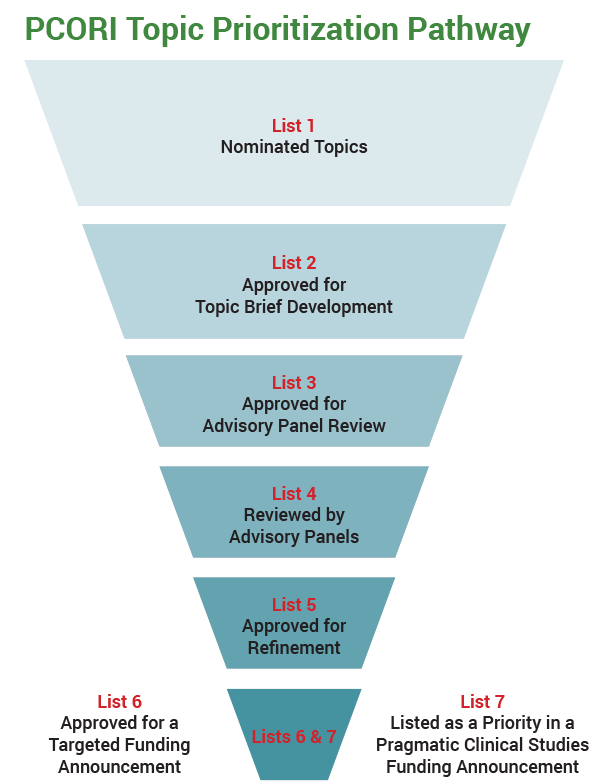
Our staff, invited stakeholders and topic experts, and members of our Board of Governors and Methodology Committee use formal review criteria as they take part in the six-step process to ensure that questions are important and well-suited for patient-centered CER. We begin with questions submitted by patients and other stakeholders through our website or other means. During the first step, PCORI staff and topic experts decide which of the topics suggested are high priority and meet specific review criteria (List 1). The staff may propose CER questions on a submitted topic. In the second step, our Science Oversight Committee (SOC)—made up of members of PCORI’s Board and Methodology Committee—determines which of the questions vetted by the staff fit our research strategy (List 2). For example, questions about surgical recovery services and care addressing substance abuse have recently been approved.
Setting Priorities
In the third step, we prepare short summaries of previous research, point out gaps in evidence, and recommend appropriate CER questions. Then the SOC reviews the summaries and selects questions for discussion by a multi-stakeholder Advisory Panel (List 3).
During the fourth step, the Advisory Panel discusses the topic briefs and ranks proposed questions according to review criteria, such as the patient-centeredness and the potential impact of the research. Currently, about 20 questions are on the list of topics reviewed by advisory panels and under active consideration (List 4). Others have moved to the next phase.
In the fifth step, the SOC then decides which of those questions will proceed (List 5). Staff then assemble a workgroup made up of topic experts and healthcare stakeholders, including patients, caregivers, clinicians, and researchers, to refine the research questions and decide which are the most important. On June 9, workgroups prioritized six sets of questions on topics that included chronic pain, depression, and new oral anticoagulants.
Creating a New Funding Announcement
Finally, staff and the SOC assign the highest-priority questions to either a targeted funding announcement (List 6), or the list of suggested topics for pragmatic clinical studies (List 7). Generally, a topic is recommended for a targeted funding announcement when we expect the studies proposed to require a higher level of funding due to their size or complexity. Targeted funding announcements typically seek a cluster of different studies about a single topic.
The Board votes on whether to approve the questions recommended for targeted funding. Staff then prepare an announcement, which we post in Funding Opportunities on our website. To date, we have released eight targeted funding announcements, each focusing on a different topic, including coronary artery disease, hypertension, hepatitis C, and care transitions. The priority-setting process has also yielded research questions, covering more than 20 other health problems, that we have included in funding announcements for pragmatic clinical studies.
What Topics Interest You?
If you are interested in a particular research topic—perhaps because it addresses a medical condition that you or a relative are living with—feel free to send us your thoughts about it or read what other people think about it. We welcome comments on the topics in our prioritization pathway, or you can submit a research question of your own. We are looking for suggestions of CER questions that will result in practical information to help patients and other stakeholders make informed decisions about their health care and health outcomes.
For more information about the types of research PCORI funds, please see Research We Support, and to see what PCORI has already funded, please visit our Funding Awards web page.