Looking Back and Looking Ahead
With holiday celebrations behind us and the new year under way, I’d like to share some thoughts about PCORI’s accomplishments over the past 12 months and some of our plans for 2020. Although I only recently assumed the role of Interim Executive Director, it’s clear to me that 2019 was an important year for PCORI with many substantial achievements.
PCORI Funding Reauthorized
Topping the list of 2019 milestones was last month’s reauthorization of our funding for another 10 years. This is a gratifying endorsement of the value of PCORI’s work to date. It also was an expression of support for the promise of what we can achieve as we continue funding research to determine which care options work best for which patients, based on outcomes important to them. There are many people to thank for this critical achievement, including our supporters in Congress, our Board of Governors, and PCORI’s remarkable staff. Most importantly, we are in debt to the many stakeholders we serve, who have guided our past work and enthusiastically endorsed its continuation.
This past year also marked the retirement of PCORI’s founding Executive Director Joe Selby, MD, MPH, which led to my taking on the interim position until a new permanent Executive Director is named. It’s been my honor to carry on the pioneering work that marked Joe’s eight-year tenure as PCORI’s leader.
Generating Evidence to Improve Healthcare Decision Making
Last year we further expanded our portfolio of studies designed to improve patient care and outcomes for many of the conditions that impose the highest burdens on patients, their families, and the healthcare system. Areas of research funded in 2019 include disease-specific topics and studies on ways to improve mental health care, reduce suicide risk, and enhance prenatal care.
By the end of 2019, we had committed $2 billion to fund more than 600 research studies and another $522 million for projects to speed the uptake of results in practice, improve the methods for how comparative clinical effectiveness research (CER) is conducted and the infrastructure to support it. We also funded nearly 800 projects to engage more patients, caregivers, clinicians, and other stakeholders in research.
We are particularly proud to be funding more than 40 pragmatic clinical trials, 10 of which began enrolling patients in 2019. These studies, which are all taking place in real-world clinical environments, test the effectiveness of widely used healthcare interventions or methods of care delivery. This type of research avoids the problem of more classic randomized trial design of uncertain applicability to everyday clinical practice and builds the rigor necessary to provide definitive answers. Many of our trials are still early in implementation, but I am confident that they will lead to important practical results that directly impact patients.
To date, we’ve made the results of nearly 300 of our funded studies available through summaries on our website.
Moving Results into Practice
Our mission is to not only fund research, but to move the results of that research into the real world where patients can benefit. Several projects illustrate the practice-changing potential of implementing the results of the research we fund. A few examples:
A project led by Shreya Kangovi, MD, MS, at the University of Pennsylvania developed a community health worker program that helped patients with multiple chronic conditions set and achieve goals for improving their health. A Dissemination and Implementation (D&I) award is now expanding IMPaCT, the Individualized Management for Patient-Centered Targets, to 4,000 patients in Delaware, North Carolina, and Pennsylvania.
 Shreya Kangovi, MD, MS, left, and Keysha Brooker, MSW, a community health worker, speak at the 2019 PCORI Annual Meeting. Kangovi is the founder of the IMPaCT program.
Shreya Kangovi, MD, MS, left, and Keysha Brooker, MSW, a community health worker, speak at the 2019 PCORI Annual Meeting. Kangovi is the founder of the IMPaCT program.
We also funded a study by David Kent, MD, MS, and his Tufts Medical Center team that developed a prediction tool to help clinicians identify which patients are most likely to develop diabetes over three years. Through a D&I award, the tool is being spread to two American Medical Group Association health systems in Pennsylvania and St. Louis, Missouri.
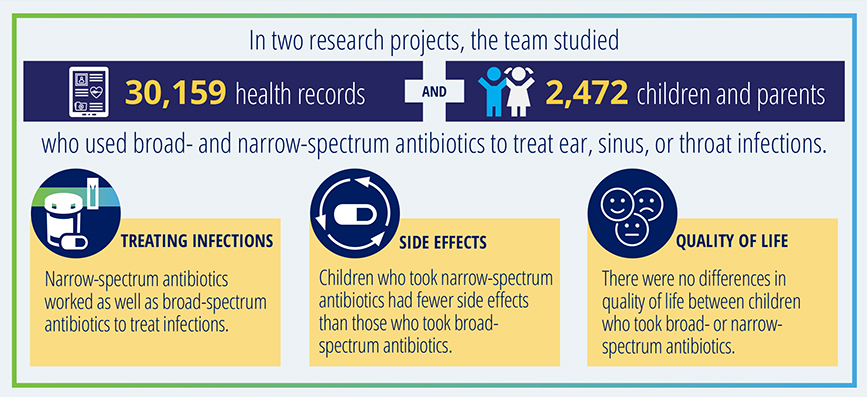
We were pleased to give a D&I award last year to Jeffrey Gerber, MD, PhD, whose Final Research Report on his PCORI-funded study on antibiotic use in children is currently the most downloaded such report on our website. Gerber’s study found that narrow-spectrum antibiotics are just as effective as broad-spectrum alternatives for treating acute respiratory tract infections in children but have a lower risk of side effects. This project will use tested strategies to improve antibiotic prescribing for more than 350,000 children across five health systems in three states.
 Jeffrey Gerber, MD, PhD, received a Dissemination and Implementation award for his project that will aim to improve antibiotic prescribing for more than 350,000 children.
Jeffrey Gerber, MD, PhD, received a Dissemination and Implementation award for his project that will aim to improve antibiotic prescribing for more than 350,000 children.
Strengthening How Research Is Done
At PCORI, we believe that involving patients, caregivers, clinicians, and other healthcare stakeholders throughout the entire research process is of great value. And in 2019, we reported on a growing body of evidence that engagement has a positive impact on research. For the March 2019 issue of Health Affairs, we reviewed information from peer-reviewed journal articles from PCORI-funded research studies. The article’s authors wrote that patients and caregivers served as active partners and contributors in many of the research projects and that engagement contributed to all aspects of research.
Among the dozens of Engagement Awards funded last year, two especially intriguing efforts are underway. The Alliance of Community Health Plans Foundation will disseminate a study from PCORI’s mental and behavioral health portfolio that uses peer-led strategies to improve access and quality of care for individuals with severe mental illness. The Alliance for Aging Research received an Engagement Award to expand its Senior Patient & Family Caregiver Network with a continued focus on patients and family caregivers of patients with Alzheimer’s disease, sarcopenia, atrial fibrillation, and chronic pain. The alliance will add two more focus areas—age-related macular degeneration and heart valve disease. The network will deliver course content through in-person and web-based training workshops, supplemented with access to a robust online community and mentorship to foster continuing education.
These achievements are extraordinary, especially given that PCORI is still a young research funder. Nevertheless, we have important work ahead. The urgency that underlies our mandate remains.
We introduced the Engagement Tool and Resource Repository for Patient-Centered Outcomes Research last year to encourage the understanding and spread of engagement practices and launched the PCORI Ambassador Center, a volunteer network of individuals dedicated to changing research.
We were pleased to see that PCORnet®, the National Patient-Centered Clinical Research Network, is evolving into a valuable resource to help conduct studies faster, more efficiently, and at lower cost than in the past. Among the most exciting news in 2019 was the announcement that the National Institutes of Health would use PCORnet® to conduct PREVENTABLE, a $90 million study to assess statins’ abilities to prevent dementia and other disabilities in older adults without cardiovascular disease. This is what we envisioned when we began developing this network of networks to leverage the power of health data, reusable research infrastructure, and unique patient partnerships.
Looking Ahead
These achievements are extraordinary, especially given that PCORI is still a young research funder. Nevertheless, we have important work ahead. The urgency that underlies our mandate remains; the need for rigorous patient-centered comparative clinical effectiveness research is as great as ever. We need better ways to move the results of that work into practice as quickly as possible and to provide stakeholders across the healthcare system with information that can better inform clinical decisions.
In 2020 we will focus on building on our accomplishments and moving quickly, with the ongoing guidance of our stakeholder communities, to show that the confidence they and Congress have expressed in us is well placed.
We recently took the first tangible steps in that direction, releasing information on a new cycle of research funding. We are inviting proposals for studies on topics that include those emphasized in the law reauthorizing our funding, addressing health concerns of people with intellectual and developmental disabilities and the worryingly high rates of maternal morbidity and mortality in the United States. This round of funding will also prioritize proposals on genetic sequencing to guide cancer treatment, peripheral artery disease, and suicide prevention. And of course, we will continue to fund research to improve how researchers conduct CER.
During the months ahead, we also will be looking at ways that we can become more efficient, improve the way we prioritize research topics, and expand our efforts to move the results of our funded research into practice.
Let me close by saying once again that, as we end one year and begin another, we are extremely grateful for the commitment of our Board and staff and the patients, caregivers, researchers, and other stakeholders we serve. We look forward to meeting the challenges of a new decade together.