PCORI’s First Advisory Panels: Celebrating a Talented and Diverse Group
In late January, we issued a broad public call for applicants for our first four PCORI Advisory Panels, the multi-stakeholder groups that will help us refine our patient-centered comparative clinical effectiveness research portfolio. We envision these panels as effective, ongoing channels to tapping the expertise of the healthcare community we serve.
Two months and 1,295 applications later, we’re confident these groups will effectively and systematically bring a range of stakeholders’ voices to our efforts to fund research that answers questions important to patients and those who care for them.
The 84 people selected to serve on our inaugural advisory panels represent the collection of experiences and perspectives critical to helping us prioritize the patient-centered topics that we expect researchers will study. We’ve posted the names, affiliations and brief biographies of our confirmed panel members, along with the stakeholder groups they represent.
The makeup of these panels is a direct reflection of the healthcare community’s dramatic response to our open call for applicants. More than 1,000 people responded, some applying to serve on more than one panel. As a result, we were able to fill each panel with 21 individuals who bring the diversity of background, expertise and focus we need to do the best job we can.
We established these panels to support three of our scientific programs – Assessment of Prevention, Diagnosis, and Treatment Options; Improving Healthcare Systems; and Addressing Disparities – with a fourth focused on Patient Engagement. Their tasks: to help us refine and prioritize research questions, provide needed scientific and technical expertise, offer input on other issues relevant to our mission, and help us model meaningful patient and stakeholder engagement efforts across all of our work.
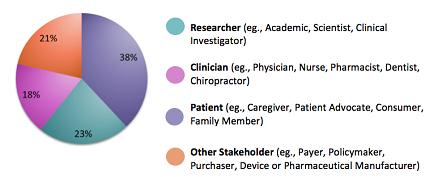
Given our overall emphasis on patient-centeredness, it’s fitting that the highest share of panelists selected across all four groups are patients, caregivers or patient advocates (38%). Representatives of this community are, appropriately, concentrated in our Advisory Panel on Patient Engagement accounting for 13 of the 21 members of that group.
Nearly one-quarter (23%) of our 84 panelists are researchers. Another 18% will provide a clinician perspective, including the views of physicians, nurses, integrative healthcare practitioners, and physician assistants. Other stakeholder perspectives represented include those of industry (pharmaceutical, device, biotech, diagnostic manufacturers), at 6%, payers (also 6%), policymakers (4%), healthcare systems (4%), and purchasers (2%).
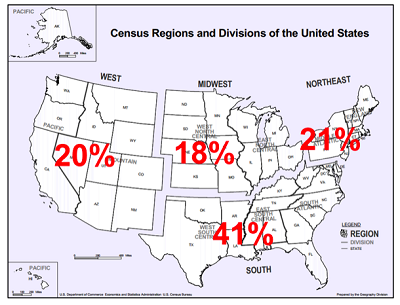
Panelists represent a range of racial and ethnic backgrounds; more than 35% of those who shared this information self-identified as a member of a minority group. There is geographic diversity as well; each area of the country is represented by at least 15 panelists, with Southern states contributing the most (34). This region includes the Washington, D.C., area, where many advocates and professional stakeholders work and reside.
Our advisory panels will soon start to focus on fulfilling the roles outlined in their charters. Although not policy-making bodies, the panels are critical to our commitment to meaningfully factor stakeholder input into the process of developing and refining our research portfolio. The panels that correspond to three of our National Priorities for Research –Assessment of Prevention, Diagnosis, and Treatment Options; Improving Healthcare Systems; and Addressing Disparities – will help us prioritize research questions and provide ongoing feedback on evaluating and disseminating the results of the studies we fund. The Patient Engagement panel, meanwhile, will begin to review best practices and consider several new opportunities to help us more effectively involve this community in our research efforts.
All panels will begin their work on April 19-20 here in the Washington, D.C. area with a training session and initial work sessions. The public will be able to listen in to panel discussions through a teleconference line. We’ll post the research topics that each panel will review and invite comments via email for the panels and PCORI staff to consider factoring into their work. Watch our website for more details.
We’re excited about our exceptional group of advisory panelists. But we know those who were not selected also are highly qualified and valuable resources for guidance on our work. So we hope they'll consider applying for one or more of the advisory panels we plan to add in the future, including those addressing Clinical Trials and Rare Diseases, as required in our authorizing legislation. We’ll fill future panels through a similar open application process.
And as pleased as we were to see such a robust response to our initial call for applicants, we want to hear from even more of you, across all stakeholder groups, to be sure that the healthcare communities we serve have a place at the table. In meantime, there are many other ways you can participate in our work, by evaluating the research proposals we receive, attending our workshops and roundtables, or suggesting a research question for us to consider for funding.
Learn more about these opportunities at our website's Get Involved section. And as always, please feel free to send us any additional comments on how we can do the best job of meaningfully engaging patients and other stakeholders in our work.
Beal served as PCORI’s Deputy Executive Director and Chief Officer for Engagement from November 2011 – March 2014