On Target: Continuing to Focus PCORI's Research Portfolio
When PCORI began funding comparative clinical effectiveness research (CER) two years ago, we issued broad funding announcements seeking the best ideas of researchers and their partnered stakeholders. We looked for comparative studies conducted in everyday settings that could change current practice and improve outcomes that matter to patients. We didn’t say anything further about the questions or clinical areas—only that proposals must be aligned with our broad National Priorities for Research and Research Agenda. We reviewed applications using our five review criteria.
From the start, though, we have said that we would continue focusing our research portfolio over time. We spent much of 2013 establishing PCORI’s infrastructure for gaining patient and stakeholder input into the topic nomination and prioritization process. We established multi-stakeholder Advisory Panels for three of our five priorities. We also established contracts for the rapid generation of topic briefs and put into place processes for studying the landscape of studies funded by others. Our research portfolio now includes the broad calls for proposals described above, targeted solicitations requesting proposals on specific questions identified through the input of patients and our other key stakeholders, and a solicitation for large pragmatic studies—head-to-head studies addressing one of a list of topics, again identified through stakeholder input.
In this blog, I’m pleased to provide a year-end update on our evolving approach to funding research, as described recently by PCORI Science Oversight Committee Chair Christine Goertz, DC, PhD, at our December 8 Board of Governors meeting.
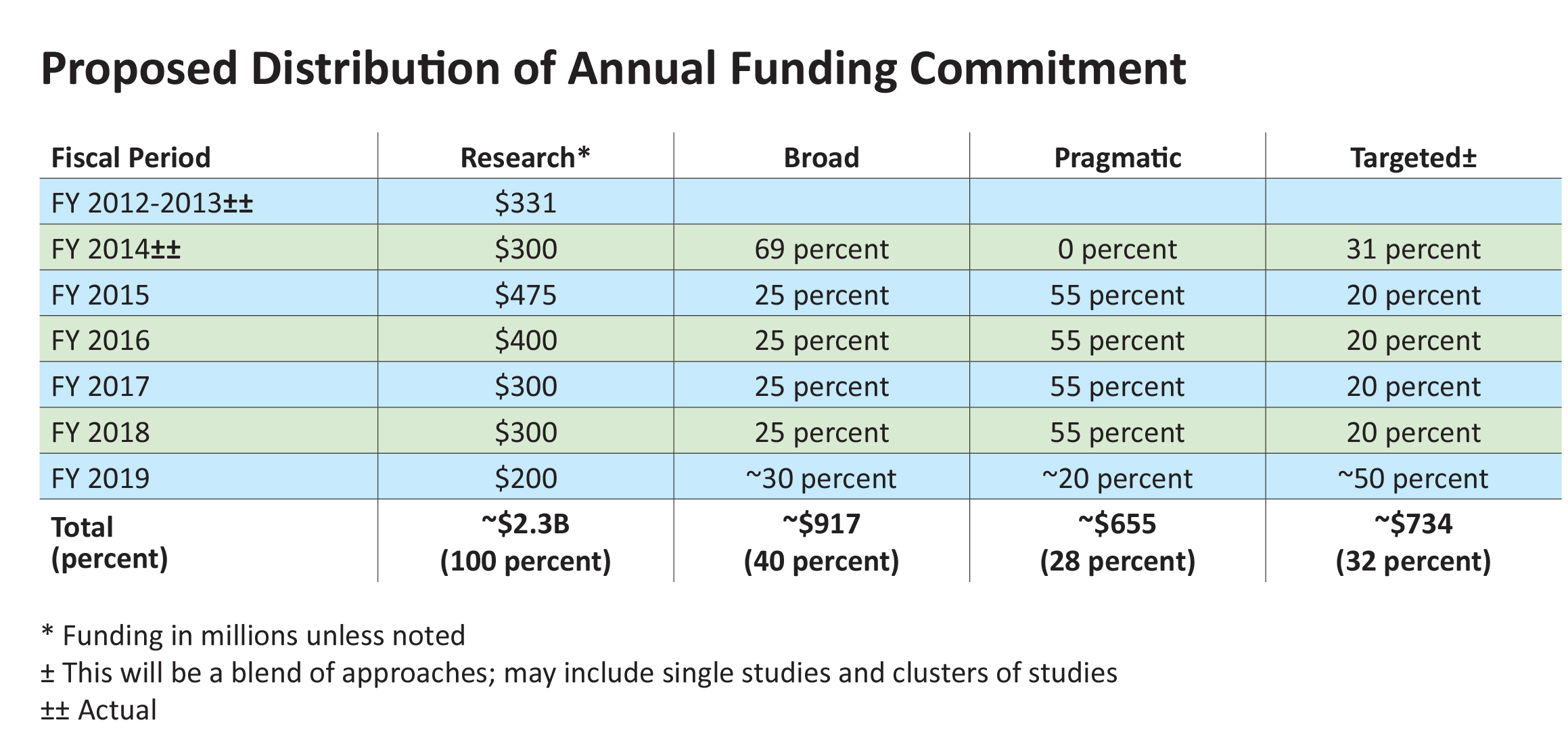
The bottom line—we’ll continue to issue broad funding announcements inviting smaller projects on a range of topics, but the amount of funding dedicated to them will decrease in future years. At the same time, we propose to continue increasing the number of larger, more targeted research projects we support and the proportion and amounts of funding dedicated to them.
Continue, but Taper, Broad Research Funding
We continue to value our traditional broad funding announcements. They yield a diverse set of proposals and represent the research community’s most creative thinking in areas of high interest to patients and other healthcare stakeholders. These awards have also served as a valuable path for supporting young scientists doing important work. We’ve funded 268 such studies for a total of more than $400 million, including clusters of studies addressing cross-cutting themes, such as shared decision making, patient navigators for care coordination in chronic illness, palliative care, and case management in vulnerable populations.
Examining the yield of these solicitations, we’ve concluded that larger clinical studies will be better suited to support the head-to-head comparisons identified as high priority by stakeholder input. To allow funding of more of these larger studies, we’ll taper the funds committed through broad funding announcements. However, these announcements will continue to be released twice yearly. We will evaluate and build on the existing portfolio of such projects and strategically manage the clusters of related studies within the portfolio. Through our advisory panels and other engagement activities, we’ll identify gaps in knowledge within the portfolio and seek funding proposals to address these gaps.
Expand Our Large Pragmatic Studies Initiative
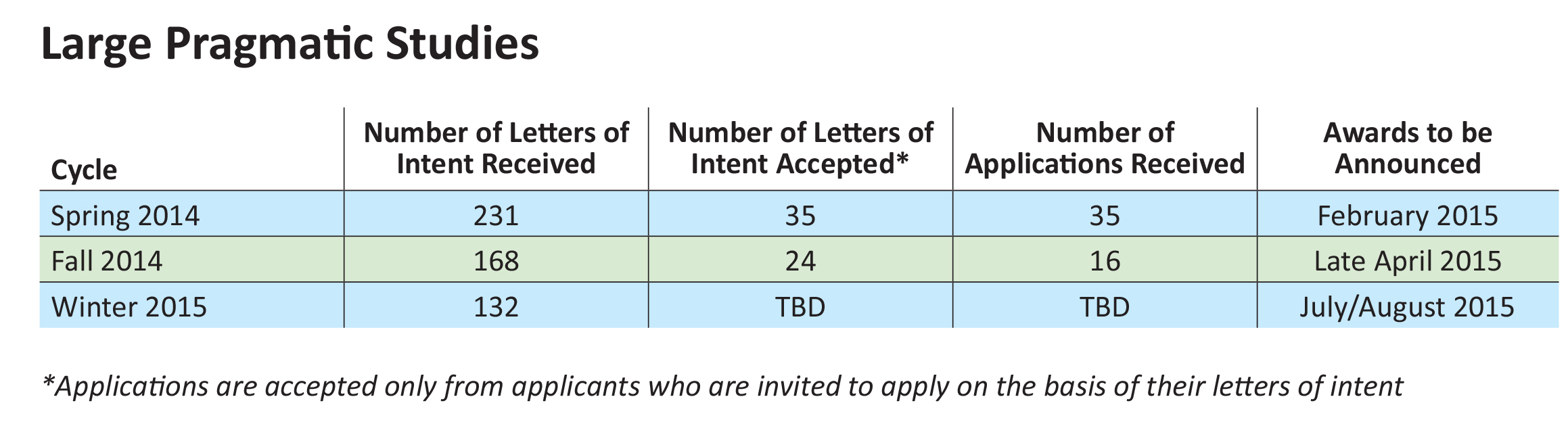
Our Large Pragmatic Studies to Evaluate Patient-Centered Outcomes initiative, launched in early 2014, is our process for funding pragmatic clinical trials, large simple trials, and large-scale observational studies comparing two or more options for addressing a major health problem. We plan to expand this promising program and are looking forward to announcing the first round of awardees in early 2015.
We’re convinced that these studies can yield transformational evidence on topics that reflect nationwide priorities for patient-centered outcomes research as identified by our stakeholders, the Institute of Medicine, and the Agency for Healthcare Research and Quality (AHRQ), and others.
These studies must be head-to-head comparisons of care approaches that include broadly representative patient populations and are large enough to provide useful evidence of how well different approaches work in different patient subgroups. As with our other funded projects, we require that relevant patient, professional, payer, or purchaser organizations play active roles in these studies, such as being research partners or serving on advisory groups.
We anticipate two upcoming funding cycles during 2015 and 2016 under this initiative, in addition to the three already released. We plan to have as many as 45 such studies under way by late 2016.
Continue to target key topics, more comprehensively
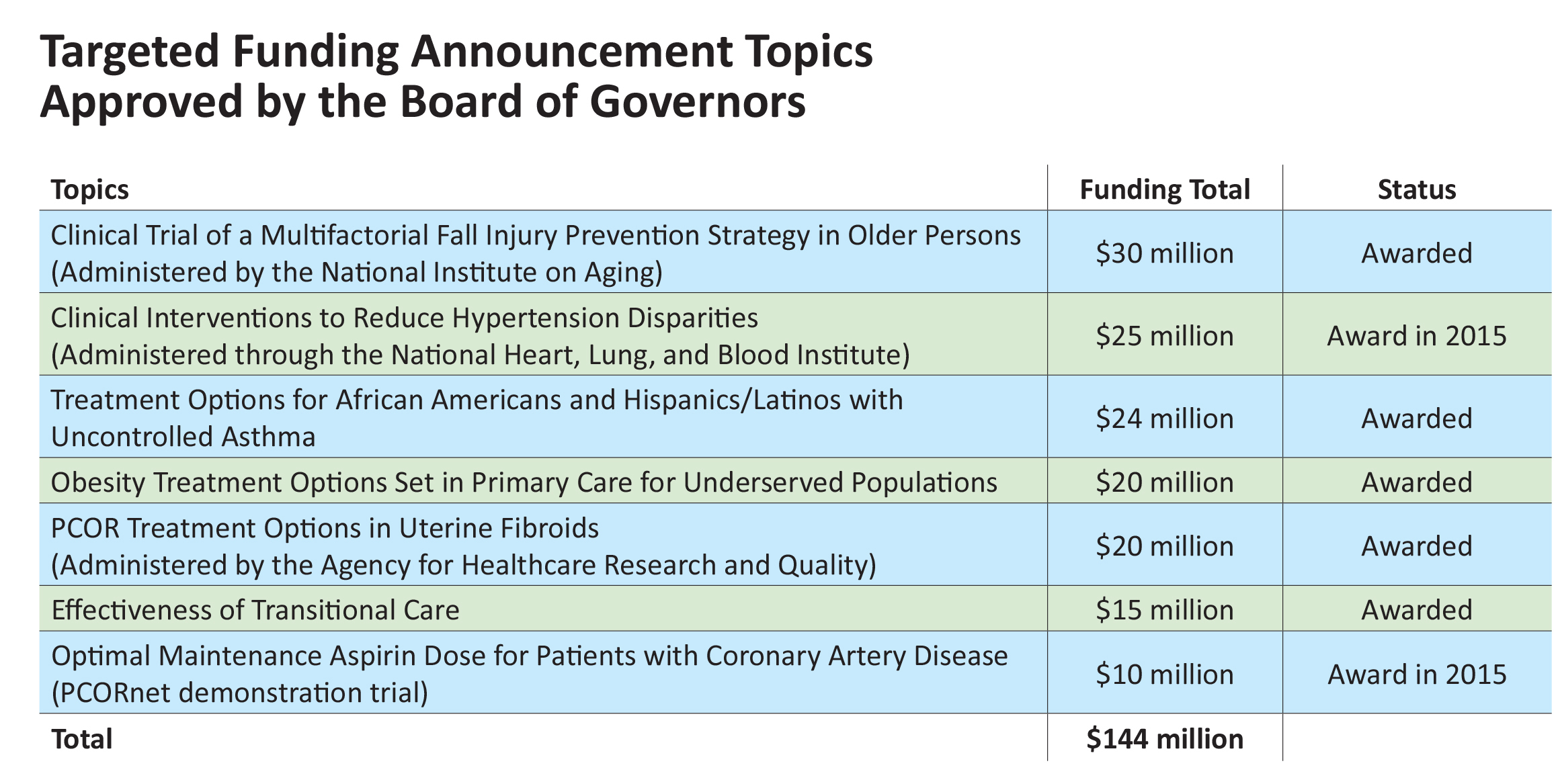
In 2013, we started issuing “targeted” funding announcements, which seek research proposals on the single, highest-priority questions identified through our topic generation and research prioritization process. To date, we’ve funded seven targeted announcements totaling $144 million in funding, including three administered by the National institutes of Health or AHRQ and our first pilot study under PCORnet, our National Patient-Centered Clinical Research Network (see below). Other targeted topics in our pipeline include one just approved by our Board—diagnosis and treatment of hepatitis C virus infection.
We’ll continue to issue targeted announcements, based on guidance provided by our stakeholders. As we manage this growing portfolio, we expect to identify a small number of key topics where added investment could make a lasting difference in care and outcomes. We will issue specific announcements for ancillary or additional studies related to these topics. Sometimes, funding may go to one additional large study; in other cases, funding could support a cluster of smaller, but related studies.
We see this new direction as providing even more opportunities to build or enhance partnerships with key stakeholder groups to ensure that the studies will have the greatest impact possible. We’re working on a detailed process for how we’ll identify these topics.
Leverage PCORnet
The last piece of our research strategy framework focuses on making use of PCORnet, the national patient-centered health data infrastructure we’re developing to boost the country’s capacity to conduct outcomes research more quickly and less expensively. This collaboration between data networks within large health systems and patient groups is designed to facilitate randomized trials, observational studies, and other types of research in real-world settings. We will soon launch within PCORnet a demonstration of randomized CER—the targeted trial mentioned above, which will investigate the optimal maintenance dose of aspirin for patients with coronary artery disease.
PCORnet is to be “research ready” by late 2015, and we welcome those who will apply for funding under the approaches above to use it in their work.
Looking Ahead
The research framework I’ve outlined builds on the investment of public funds entrusted to us; leverages our relationships with stakeholders, our multi-stakeholder advisory panels, and their research prioritization efforts; and harnesses the momentum created by our programs. It also maintains our flexibility to respond to opportunities or critical national needs, enhances our partnership opportunities, and provides paths to focus our resources to maximize impact on practice and outcomes.
Of course, there are risks, as with any strategy. But as a learning healthcare organization, our Board of Governors and staff are committed to evaluating our work on an ongoing basis and adjusting our approach as needed. That’s what we’ve done since our inception, and it remains one of our guiding principles.
I think this makes a compelling blueprint for the next five years. As we follow it, I hope you’ll keep an eye on our progress.