Welcoming Our New Advisory Panel Members
It was two years ago this week that we convened our first multi-stakeholder PCORI Advisory Panels, groups brought together to help us guide our research funding activities. During that short time, our original slate of four panels has grown to seven, and their impact on our work has been clear and invaluable.
This week, we took the latest step in ensuring that we stay in touch with the broad healthcare community as we continue to refine our research agenda. Our Board of Governors approved 70 people to serve on our advisory panels—47 new members and 23 reappointments.
 Overall Composition of Current Advisory Panels
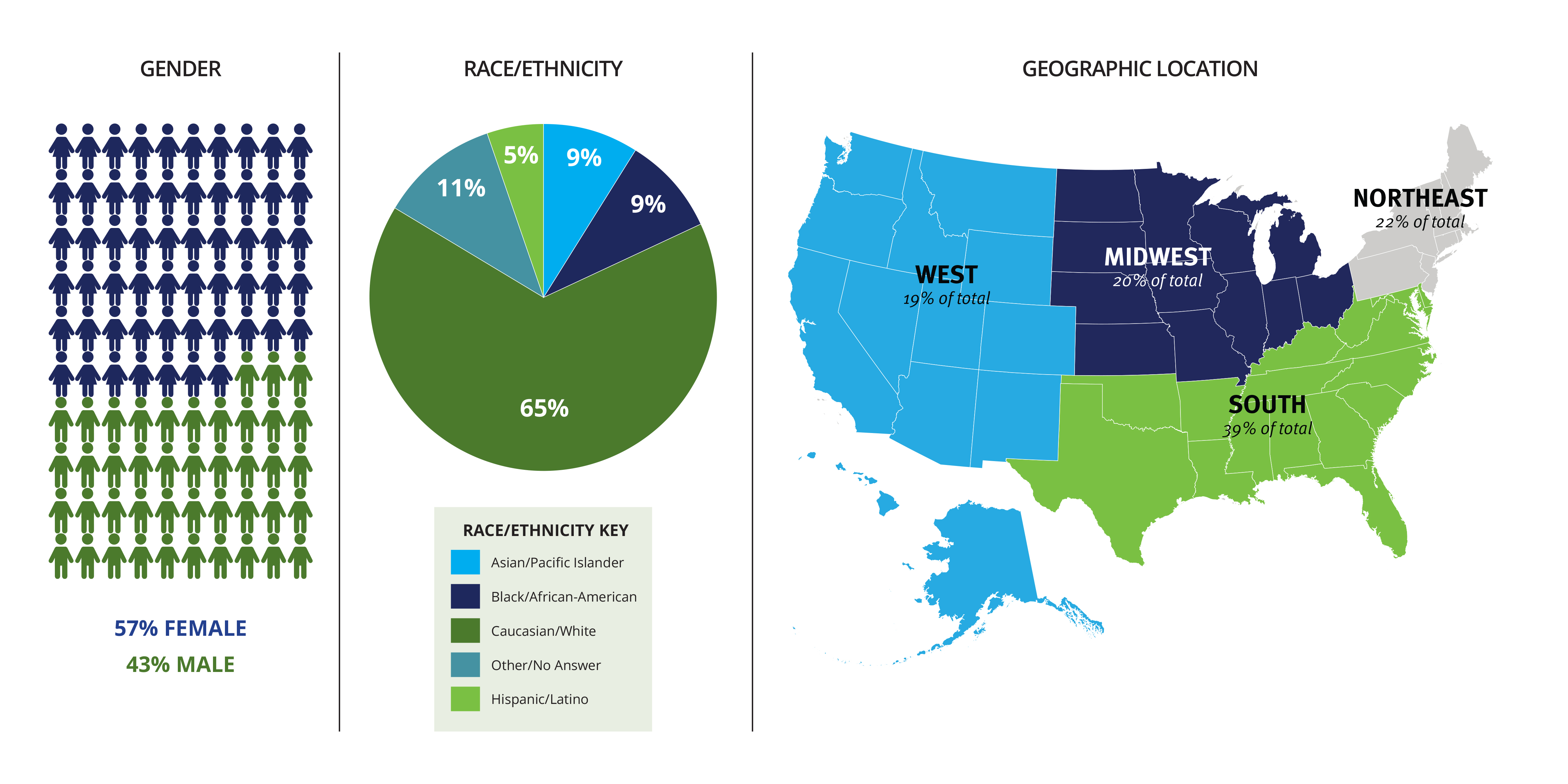
Overall Composition of Current Advisory PanelsClick the graphic for a larger version
Among the new members are 21 people who make up our seventh and newest panel—the Advisory Panel on Communication and Dissemination Research. The other new members replace panelists who are rotating off five of the six previously established panels. The overall composition of our panels is now 57 percent female with a broad representation of racial and ethnic groups and geographic locations.
Our Advisory Panels
Our advisory panels—each with 10 to 21 members—are charged with advising and providing recommendations to our Board, Methodology Committee, and staff on planning, developing, implementing, and enhancing our work as we build a portfolio of patient‐centered comparative clinical effectiveness research. Each panel includes representatives of healthcare stakeholder communities, such as patients, caregivers, researchers, clinicians, payers, and industry.
Since their creation, each of the panels—with the exception of the Advisory Panel on Communication and Dissemination Research—has convened four to seven times. These meetings are typically open to the public via webinar/teleconference. They often include discussions to prioritize topics for research funding and to consider emerging and ongoing issues related to the panel's area of concern. You can find summaries on each panel's Meetings & Events pages.
Our Multi-Stakeholder Advisory PanelsCorresponding to Our National Priorities and Research Agenda: Cited in Our Authorizing Law: To Ensure Patient Engagement and Patient‐Centeredness: |
The Selection Process
Some applicants apply voluntarily, while others are nominated by another individual or an organization. During this recent cycle, more than two dozen private individuals and organizations made nominations, and six of those nominees—recommended by the American Academy of Pediatrics, Infectious Diseases Society of America, America's Health Insurance Plans, the American Association on Health and Disability, PhRMA and Biotechnology Industry Organization, and the American Heart Association—were selected to join an advisory panel.
The application process includes submission of a personal statement for volunteers or letters of endorsements for nominees, in both cases describing the individual's background and experience. We then conduct a three-step review and selection process, before our Board reviews and approves the appointments.
Applications are accepted year-round, and those received too late for a given cycle are retained for consideration for future openings.
Learn More About the Composition of Each of Our Current Advisory Panels
Meet Some of Our New Panelists
The new panelists approved yesterday were selected from 770 applicants. Applications came from 46 US states and Canada and Sweden. The applicants represented a broad range of racial, ethnic, and age groups and academic qualifications; nearly 60 percent were women.
We are pleased to welcome both panel members chosen for their professional expertise and for their personal experiences. Among the members of the newly created Advisory Panel on Communication and Dissemination Research are Sandi W. Smith, PhD, director of the Health and Risk Communication Center and a professor in the Department of Communication at Michigan State University, and Cornell Wright, MPA, director of the North Carolina Office of Minority Health and Health Disparities.
The new 21-member panel also includes about a dozen patient, caregiver, and consumer advocates, including LaRita B. Jacobs, MA, a freelance medical and human-interest journalist, and Danny van Leeuwen, MPH, RN, CPHQ, a nurse leader and public speaker who maintains a health blog with more than 2,000 registered users.
Meanwhile, as part of PCORI's commitment to ensuring the highest patient engagement standards and a strong culture of patient-centeredness, our Advisory Panel on Patient Engagement welcomes seven new members. Among them are Jane Perlmutter, PhD, MBA, a breast cancer survivor who has been involved in health advocacy—with a special interest in clinical trials—for 30 years, and Jimmy Lin, MD, PhD, MHS, founder and president of the Rare Genomics Institute.
What's Ahead
We’re confident that our advisory panels effectively and systematically bring a range of stakeholders' voices to help us fund research that answers questions important to patients and those who care for them.
Five of the seven panels, including the new panel, will hold their Spring 2015 meetings at the end of May and beginning of June. More information on each of those meetings, including dates, can be found on our Upcoming Events page.
We'd like to thank everyone who applied or made nominations. Learn more about the new and returning panelists, and the stakeholder groups they represent, on the biography page of each panel.
Related Content- News Release: PCORI Names 21 Inaugural Members of New Advisory Panel on Communication and Dissemination Research